Current and Emerging Treatment
Vascular endothelial growth factor (VEGF) plays a critical role in promoting angiogenesis and vascular leakage, and several different anti-VEGF agents have been studied in the management of DME, including aflibercept, bevacizumab, pegaptanib, and ranibizumab, and the same agents are now used for PDR;1 with some being studied in NPDR patients, as well. Anti-VEGF agents exhibit important differences in their sites of activity, formulation methods, binding affinities, and biological activities.2-6
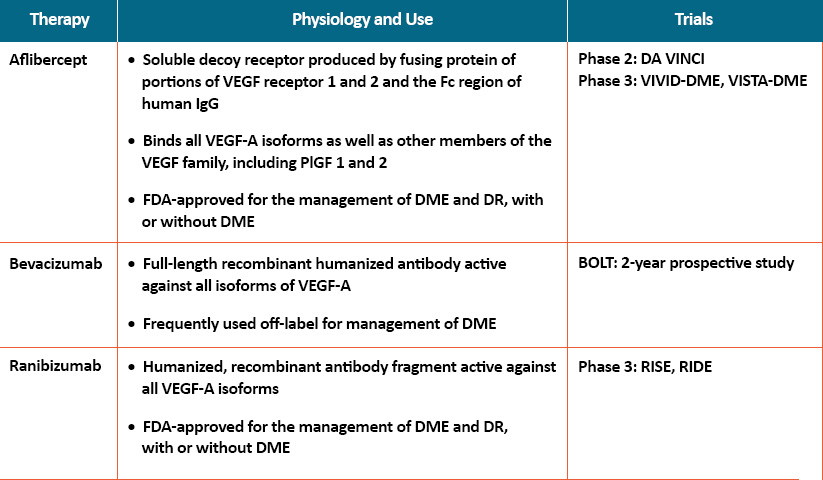
- Aflibercept is a soluble decoy receptor produced by fusing protein of portions of VEGF receptor 1 and 2 and the Fc region of human IgG. It binds all VEGF-A isoforms as well as other members of the VEGF family, including PlGF 1 and 2 that have been shown to determine excessive vascular permeability. The DA VINCI phase 2 double-blind clinical trial compared different doses and dosing regimens of aflibercept in patients with DME to laser photocoagulation. A significant improvement in best-corrected visual acuity (BCVA) was achieved at week 24 and was maintained or enhanced at week 52 in all aflibercept arms. There was a higher incidence of increased intraocular pressure (IOP) and eye pain in the aflibercept group compared with laser. Aflibercept was approved by the US FDA for use in DME patients based on results from 2 phase 3 trials: VIVID-DME and VISTA-DME. A decision by the FDA to approve the use of aflibercept in patients without DME (ie, NPDR and PDR) was made in May 2019.
- Bevacizumab is a full-length recombinant humanized antibody active against all isoforms of VEGF-A and is frequently used off-label for management of DME. Results from the 2-year prospective BOLT study comparing bevacizumab versus laser in persistent DME showed that the mean gain in ETDRS letters was 9 and 2.5 letters in the bevacizumab and laser groups, respectively; an improvement of 10 or more letters was seen in 45% and 7% of the 2 arms, respectively. Post-hoc analysis of injection frequency revealed the only determinant of fewer injections in the second year was a better baseline visual acuity; in addition, eyes with subretinal detachment required more injections than diffuse and cystoid edema. There is some concern among clinicians about an increase in major cardiovascular events using bevacizumab, and while substantial data is lacking, it may need to be avoided in high-risk patients.
- Ranibizumab is a humanized, recombinant antibody fragment active against all VEGF-A isoforms. It is currently FDA-approved for the management of DME and DR, with or without DME. In the RISE and RIDE phase 3 clinical trials, ranibizumab was given every month to patients with DME and compared with sham treatment; all patients were allowed to receive focal laser treatment at the discretion of the treating physician after 3 months. After 2 years, 33.6% to 44.8% of ranibizumab-treated patients vs 12.3% to 18.1% of sham-treated patients achieved the primary endpoint, which was a gain of at least 15 letters of vision, and 54.4% to 60% of ranibizumab recipients achieved 20/40 or better vision vs 34.6% to 37.8% of sham-treated patients. These gains in vision were sustained through a third year of Diabetic Retinopathy treatment in the ranibizumab group. Higher incidence of increased IOP was reported in the ranibizumab arms; there were no consistent differences in systemic adverse events between ranibizumab and laser or placebo.
One- and 2-year results from a comparative effectiveness trial of aflibercept, bevacizumab, and ranibizumab showed that all 3 anti-VEGF groups had improvement in visual acuity (VA) from baseline with a decreased number of injections in year 2. VA outcomes were similar for eyes with better baseline VA. Among eyes with worse baseline VA, aflibercept was more effective at improving vision at 1 year,7 and had superior 2-year VA outcomes compared with bevacizumab.8
Distinguishing Patient Populations and Outcomes of Recent Pivotal Studies
The recent Phase 3 PANORAMA study evaluated the efficacy and safety of aflibercept, which is now approved for patients with DME and DR, with or without DME. PANORAMA studied patients with moderately severe to severe NPDR at every 8-week (q8) and every 16-week (q16) interval injections versus sham injections. At 52 weeks, 80% and 65% of patients on the q8- and q16-week intervals, respectively, experienced a two-step or greater improvement from baseline on the Diabetic Retinopathy Severity Scale (DRSS), compared to 15% of patients receiving sham injections, after an initial monthly dosing period.9
There is 1- and 2-year data evaluating bevacizumab, which is used off-label, in patients with DME based on DRCR.net Protocol T.
As noted, ranibizumab received an indication for use in patients with DR with or without DME based on RISE and RIDE studies, which evaluated patients with DME, and Protocol S, which evaluated patients with PDR, with or without DME, compared to the use of laser photocoagulation, where mean visual acuity letter improvement at 2 years was +2.8 in the ranibizumab group versus +0.2 in the laser photocoagulation group.10
Emerging Targets
Several novel treatments in pre-clinical or clinical trials have the potential to meet current needs in DME and/or DR treatment. These include corticosteroid-releasing implants, which may be particularly useful in pseudophakic patients, and treatments targeting the kinin–kallikrein system (KKS). Stabilizers of vasoprotective Tie-2 signaling have yielded promising results in pre-clinical development, and represent a strategy for preventing disease-associated endothelial dysfunction; the TIME-2 trial evaluated a Tie-2 activator in combination with an anti-VEGF therapy with favorable results. Also, clinical trial data based on targeting the inflammation characteristics of early disease with members of multiple drug classes including NSAIDs, immunosuppressants, and antibiotics are being evaluated.11
References
- Solomon SD, Chew E, Duh EJ, et al [2017 ADA Position Statement]. Diabetic retinopathy: A position statement by the American Diabetes Association. Diabetes Care. 2017;40(3):412–418.
- Bandello F, Casalino G, Loewenstein A, Goldstein M, Pelayes D, Battaglia Parodi M. Pharmacological approach to diabetic macular edema. Ophthalmic Res. 2014;51:88-95.
- Ford JA, Lois N, Royle P, Clar C, Shyangdan D, Waugh N. Current treatments in diabetic macular oedema: systematic review and meta-analysis. BMJ Open. 2013;3:e002269.
- Thomas BJ, Shienbaum G, Boyer DS, Flynn Jr HW. Evolving strategies in the management of diabetic macular edema: clinical trials and current management. Can J Ophthalmol. 2013;48:22-30.
- Lim JI. Unmet needs in diabetic macular edema. US Ophthalmic Rev. 2011;4(1):101-104.
- Tarr JM, Kaul K, Kohner EM, Chibber R. Diabetic retinopathy: major unmet medical challenge. Endocrinol Studies. 2011;1(e7):28-36.
- The Diabetic Retinopathy Clinical Research Network [DRCR.net]. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015; 372:1193-1203.
- Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351-1359.
- Wyckoff CC. What the latest DR data means for patients. Ophthalmol Manag. 2019;23. https://www.ophthalmologymanagement.com/issues/2019/july-2019/what-the-latest-dr-data-means-for-patients.
Accessed December 3, 2019. - Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, Antoszyk AN, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. JAMA. 2015; 314(20):2137–2146.
- Bolinger MT, Antonetti. Moving Past Anti-VEGF: Novel therapies for treating diabetic retinopathy. Int J Mol Sci. 2016;17(9):1498.
doi: 10.3390/ijms17091498.