Pathophysiology
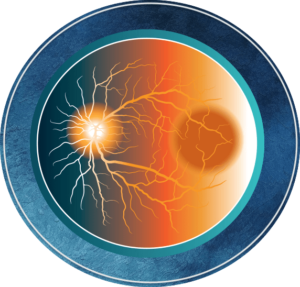
Vision loss due to DR results from several mechanisms, shown in the Figure below.1
- Central vision may be impaired by macular edema as the result of increased vascular permeability and/or capillary nonperfusion
- New blood vessels of PDR and contraction of the accompanying fibrous tissue can distort the retina and lead to tractional retinal detachment producing severe and often irreversible vision loss
- New blood vessels may bleed, ie, complicating preretinal or vitreous hemorrhage
- Vascular changes can be accompanied by damage to retinal neurons
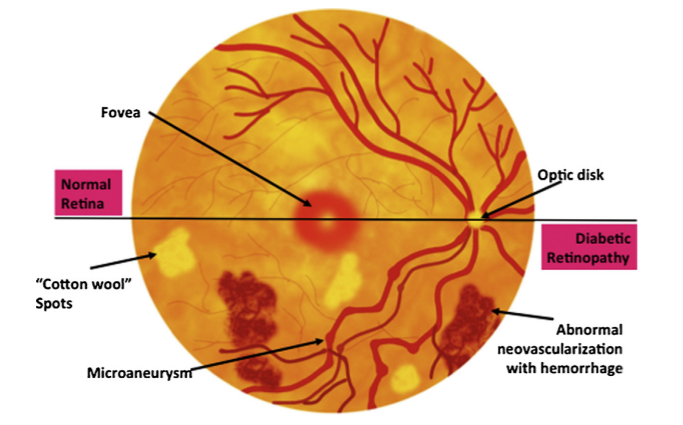
DR can be divided into 2 stages: non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR). NPDR can be further divided into mild, moderate, and severe stages.1 In general, retinopathy advances from mild nonproliferative abnormalities, characterized by increased numbers of microaneurysms. With increasing severity, there is increased vascular permeability and occlusion with progression from moderate and severe NPDR to PDR, characterized by the growth of new retinal and vitreal blood vessels,1 as noted in the Figure above and Table below.
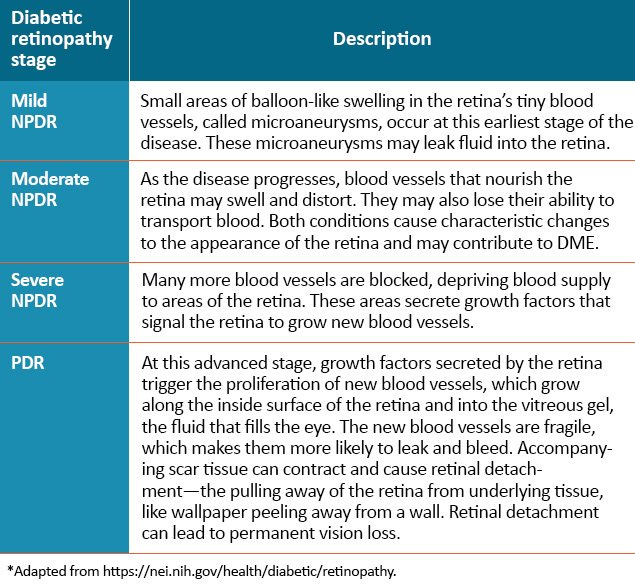
DME = diabetic macular edema; NPDR = nonproliferative diabetic retinopathy; PDR = proliferative diabetic retinopathy.
Chronic hyperglycemia induces oxidative stress through a variety of potential pathways, resulting in alterations of metabolic homeostasis within the retina. These changes can initiate compensatory responses, instigating vascular dilation and increased retinal blood flow.3-5
Pericytes — cells within retinal capillary walls that provide physical strength and biochemical support to underlying endothelial cells — are damaged and ultimately lost. Consequently, the integrity of endothelial cells can be compromised, leading to the development of localized outpouchings in capillary walls, called microaneurysms. This is the earliest clinically evident stage of diabetic retinopathy. Subsequently, the integrity of the outer blood-retina barrier is impaired, eventually resulting in the leakage of plasma into the surrounding neural tissues.3-5
Chronic hyperglycemia and oxidative stress also stimulate an inflammatory response within many cell types, including vascular endothelial cells. Increased leukocyte-endothelium adhesion may cause endothelial and neuroglial cell injury by capillary occlusion. Injury may also occur through the release of inflammatory mediators triggered by tissue hypoxia.1,3-8
Retinal hypoxia and ischemia — as well as microglial hyperglycemia — lead to the upregulation and secretion of multiple cytokines, including vascular endothelial growth factor (VEGF). Diffusible VEGF has three complementary roles, including driving endothelial cell proliferation — or angiogenesis — increasing vascular permeability, and increasing local inflammatory infiltrates.1,6-8
VEGF can promote endothelial expression of intercellular adhesion molecule-1 (ICAM-1), inciting leukocyte activation and cytokine release, which leads to additional cytokine secretion — including VEGF — with continued propagation of the inflammatory response. Chronic inflammation contributes to capillary occlusion and breakdown of the blood-retinal barriers critical for normal retinal homeostasis under physiologic conditions.1,6-8
Long-term, increased plasma leakage and continued pericyte loss can cause further breakdown of the inner blood-retina barrier, with ensuing vascular degenerative changes, such as endothelial cell apoptosis and basement-membrane thickening. Vascular swelling and structural distortion stemming from the loss of integrity in the blood-retina barrier eventually result in capillary occlusion and ischemia, increasing plasma leakage and progression of the continuum of diabetic macular edema (DME).1-8
The pathogenesis of DME is at this time poorly defined, but is believed to involve angiogenesis, inflammation, and oxidative stress.9,10 The pathophysiology of DME involves dilated capillaries, retinal microaneurysms, and loss of pericytes, with eventual impairment of the blood-retinal barrier (BRB).11,12 Breakdown of the BRB results in fluid leakage into the extracellular space, which disrupts macular structure and function on a cellular level.13 Hyperglycemia is reported to lead to capillary endothelial damage and alterations in leukocyte function;14 additionally, hyperglycemia has been reported to activate oxidative stress agents, such as advanced glycation end products and the protein kinase C (PKC) pathway.11,15 Various inflammatory mediators appear to play a role in promoting DME, including VEGF, placental growth factor (PlGF), hepatocyte growth factor (HGF) and others.10,11,16-18
References
- Solomon SD, Chew E, Duh EJ, et al [2017 ADA Position Statement]. Diabetic retinopathy: A position statement by the American Diabetes Association. Diabetes Care. 2017;40(3):412–418.
- Eshaq RS, Aldalati AMZ, Alexander JS, Harris NR. Diabetic retinopathy: Breaking the barrier. Pathophysiology. 2017;24:229–241.
- Abcouwer SF. Angiogenic factors and cytokines in diabetic retinopathy. J Clin Cell Immunol. 2013; suppl 1:1-12.
- Feng S et al. Levels of inflammatory cytokines IL-1β, IL-6, IL-8, IL-17A, and TNF-α in aqueous humor of patients with diabetic retinopathy. J Diabetes Res. 2018:8546423.
- Kowluru RA, Chan PS. Oxidative stress and diabetic retinopathy. Exp Diabetes Res. 2007;2007:43603.
- Kusuhara S et al. Pathophysiology of diabetic retinopathy: the old and the new. Diabetes Metab J. 2018;42:364-376.
- Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30:343-358.
- Wang W, Lo AC. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018;19:1816.
- Ehrlich R, Harris A, Ciulla TA, Kheradiya N, Winston DM, Wirostko B. Diabetic macular edema: physical, physiological and molecular factors contribute to this pathological process. Acta Ophthalmologica. 2010;88:279-291.
- Urias EA, Urias GA, Monickaraj F. Novel therapeutic targets in diabetic macular edema: beyond VEGF. Vision Research. 2017;139:221-227.
- Javey G, Schwartz SG, Flynn Jr HW. Emerging pharmacotherapies for diabetic macular edema. Exp Diabetes Res.
2012;2012(548732):1-12. - Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003;26:2653-2664.
- Rotsos TG, Moschos MM. Cystoid macular edema. Clin Ophthalmol. 2008;2:919-930.
- Morigi M, Angioletti S, Imberti B, et al. Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-kB-dependent fashion. J Clin Invest. 1998;101:1905-1915.
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615-1625.
- Cai W, Rook SL, Jiang ZY, Takahara N, Aiello LP. Mechanisms of hepatocyte growth factor-induced retinal endothelial cell migration and growth. Invest Ophthalmol Vis Sci. 2000;41:1885-1893.
- Miyamoto N, de Kozak Y, Jeanny JC, et al. Placental growth factor-1 and epithelial haemato-retinal barrier breakdown: potential implication in the pathogenesis of diabetic retinopathy. Diabetologia. 2007;50:461-470.
- Shams N, Ianchulev T. Role of vascular endothelial growth factor in ocular angiogenesis. Ophthalmol Clin North Am. 2006;19:335-344.